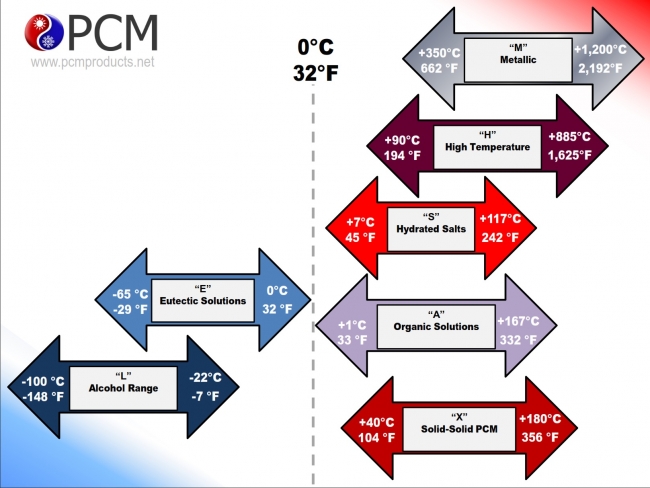
Types of Phase Change Materials
In selecting phase change materials the main phase change of interest is the solid/liquid phase change. There are other types of phase change which also store and release latent heat energy during their transition, but these are generally not practicable for most energy storage applications. For example, liquid/gas phase changes involve large changes in volume or pressure when going from the liquid to the gaseous phase, which prevent effective encapsulation.
Some materials exhibit solid/solid phase changes, in which the crystalline structure is altered at a certain temperature. These are available in limited temperature ranges.
Solid / Liquid Phase Change Materials
The solid/liquid phase change materials that PCM Products supply can be most simply divided into four categories: eutectics, salt hydrates, organic materials, and high temperature salts.
Browse the links below for more extensive descriptions of the categories:
- Sub Zero Eutectic PCMs
- Ultra Low Temperature Eutectic PCMs
- Positive Temperature Salt Hydrate PCMs
- Positive Temperature Organic PCMs
- High Temperature Salt PCMs
- Solid / Solid PCMs
- PCM variations
To be a useful PCM, a material should ideally meet several criteria:
Release and absorb large amounts of energy when freezing and melting
This requires the PCM to have a large latent heat of fusion and to be as dense as possible
Have a fixed and clearly determined phase change temperature (freeze/melt point)
The PCM needs to freeze and melt cleanly over as small a temperature range as possible. Water is ideal in this respect, since it freezes and melts at exactly 0 °C (32 °F). However, many PCMs freeze or melt over a range of several degrees, and will often have a melting point that is slightly higher or lower than the freezing point. This phenomenon is known as hysteresis.
Avoid excessive supercooling
Supercooling is observed with many eutectic solutions and salt hydrates. The PCM in its liquid state can be cooled below its freezing point whilst remaining a liquid. Some salt hydrates can be cooled to 50 °C (122 °F) below their freezing point without crystallisation occurring. This can be beneficial, for example in hot packs where a 48 °C (118.4 °F) PCM is kept as a supercooled liquid at room temperature until the hot pack is required and supercooling is broken by mechanical or chemical nucleation. However for most applications, supercooling must be kept to a minimum by the addition of suitable nucleating agents to the PCM.
Remain stable and unchanged over many freeze/melt cycles
PCMs are usually used many times over, and often have an operational lifespan of many years in which they will be subjected to thousands of freeze/melt cycles. It is very important that the PCM is not prone to chemical or physical degradation over time which will affect the energy storage capability of the PCM. Some eutectic solutions may be susceptible to microbiological attack, so must be protected with biocides. Long term stability can be a problem in some salt hydrate PCMs, unless they are modified to prevent separation of the component materials over successive freeze/melt cycles.
Be non-hazardous
PCMs are often used in applications whereby they could come in contact with people, for example in food cooling or heating applications, or in building temperature maintenance. For this reason they should be as safe to use as possible. Ideally a PCM should be non-toxic, non-corrosive, non-hazardous and non-flammable. There are many substances that behave excellently as PCMs but cannot be used due to issues over safety.
Be economical
It doesn't matter how well a substance can perform as a PCM is if is prohibitively expensive. PCMs can range in price from very cheap (e.g. water) to very expensive (e.g. pure linear hydrocarbons). If the cost of the PCM outweighs any benefit obtained from using the PCM, its use will be very limited.
Solid / Solid Phase Change Materials
Please find more information about solid / solid PCMs on this page:
Clathrates
Clathrates have been suggested as a potential group of PCMs. Clathrates essentially involve host molecules arranging themselves in such a way as to create a cage and trapping a different molecule within the cage structure. Under certain conditions it is possible to create gas hydrate clathrates where the host is water and the trapped species is a gas such as methane, natural gas, or some refrigerant gases. These clathrates look like a solid crystalline material, store large amounts of heat when they melt, and some have melting temperatures that are attractive to energy storage applications. Unfortunately, the clathrates can currently only be produced by mixing the the host and trapped species under very large pressure, and once the clathrate has melted the two species cannot easily be reincorporated, so they can only be used once as a PCM.
There are another group of organic materials that can form clathrates with water under normal atmospheric conditions and can be used through repeated freeze and melt cycles. These stable clathrates have very high latent heat values, and change phase at temperatures comparable to other positive temperature PCMs. If you would like to know more about these materials please contact us in the same manner as above.